
Gå tillbaka
Migration from low to a high Type 1 diabetes incidence country increases disease in subjects
with low genotype risk
Ahmed Delli, Åke Lernmark, Bengt Lindbland, Annelie Carlsson, Sten Ivarsson, Leif Blom, Leif Groop, Gun Forsander, Anna Kernell, Johnny Ludvigsson, Claude Marcus, Ingmar Zachrisson, Ann-Katrin Karlsson, Ingrid Kockum, Kristian Lynch, Anita Nilsson, and Lisa Engleson.
This research was accomplished for the Better Diabetes Diagnosis (BDD) study at the Clinical Research Center (CRC) of Lund University, UMAS, Malmö.
Background
The incidence of Type 1 Diabetes (T1D) varies widely according to geographic location and ethnic backgrounds. 600-fold difference in incidence rates was observed between Finland1, which has the highest incidence in the world and low incidence countries such as China and Venezuela.2 This wide difference is thought to be related to variations in genetic heritage and environmental factors.1 Genes are important determinants of T1D risk and the Human Leukocyte Antigen (HLA) class II genes are considered as necessary but not sufficient risk factors3; however, the current rapidly changing global trends suggest an important role of environmental factors. Previous studies4,5 reported an increased disease risk among children of parents who migrate from low to high incidence T1D country. However, these ”migration” studies analyzed epidemiological data only and did not study genetic and/or immunologic determinants. Sweden has the highest second incidence in the world scoring more than 40 pateints/100000/year in 2005.5 The current study examined the effect of country of origin and migration on the risk of T1D among children ≤18 years old in Sweden in relation to genetic (HLA) and immunologic (islet autoantibodies) risk factors.
Methods
Subjects were recruited from a prospective study called Better Diabetes Diagnosis (BDD) study that was initiated in may 1st 2005 and is still ongoing with 40 (95%) pediatric centers are participating nationwide. All new T1D patients diagnosed within the BDD during the period of May 1st, 2005 to October 31st, 2007 who were ≤18 years old at time of diagnosis were included in this study. Subjects were classified according to country of origin of their parents and grandparents into four groups: Swedish, non-Swedish, Swedish-Finnish and Swedish-Others and were compared with respect to HLA-risk genes and islet autoantibodies (GAD65A, IAA, IA-2A).
Results and Discussion
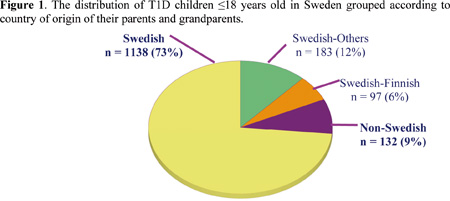
A total of 1550 (860 males and 690 females) patients were registered. 1524 (98%) patients were born in Sweden. Swedish children constituted 73% of the cohort, while non-Swedish children constituted ~9% and 18 % had mixed origins. High percent (86.5%) of cases were diagnosed before the 15th birthday. The overall incidence rate during study period was 30 (28–32)/105/year in children ≤18 years and 35 (33–37) in children ≤14 years. Non-Swedish migrant children had 2 to 3 times higher incidence rates (17/ ≤18 years and 19/ ≤14 years) than the reported rates of the majority of their countries of origin.
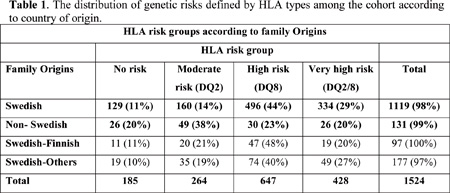
Very high (DQ8&2) and high (DQ8) risk HLA alleles were more prevalent among Swedish children while moderate (DQ2) and low (neither DQ8 nor DQ2) were more prevalent among non-Swedish children. Logistic regression showed that Swedish children had significantly higher genetic (HLA-DQ8) risk (OR=3.7, p<0.0001) and immunologic (IA-2A and IAA) risk (OR=2.0, p<0.001 and OR=1.6, p<0.05 respectively) than non-Swedish children. Mixed-origins children showed minor differences from Swedish children. Migration to a diabetogenic country has been implicated to increase the risk of T1D among children of migrants from low-incidence country. In this study, non-Swedish children comprised 9% of T1D patients and the majority (~66%) of those children had their country of origin from south and east Europe and Middle East and North Africa. These two regions have significantly lower incidence rates than Sweden.1 However; the incidence rates of non-Swedish children (17/105/year/≤18 years and 19/105/year/≤14 years) were 2 up to 3 times higher than the reported incidence rates from the majority of their countries of origin. In order to avoid over-estimation, the reference population used to calculate the above incidence rates involved all children with ”foreign backgrounds” including children who obtained Swedish citizenship.
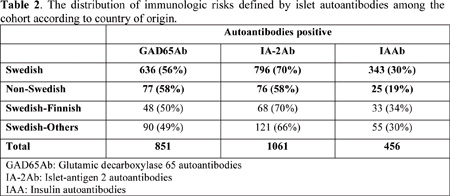
The HLA class II DQ8 and DQ2 are the main haplotypes that confer the highest genetic risk of T1D and the DQ8/DQ2 is the commonest predisposing genotype.3 In this study, Swedish children were found to have more frequent DQ8 and DQ8/2 and DQ8 in specific seems to be the main genetic determinant among Swedish children. Risk analysis using logistic regression models showed that: a Swedish child who has DQ8 carries more than three times higher risk to develop T1D than non-Swedish child (OR=3.7, p<0.0001). On the other hand, non-Swedish children had more frequent the moderate risk DQ2 haplotype than Swedish children (p<0.006 ). This may indicates that ”migrant” children may have lesser genetic predisposition than Swedish children who carry ”stronger” determinants. Similarly, Islet-antigen 2 autoantibodies (IA-2Ab) and insulin autoantibodies (IAA) were significantly more frequent among Swedish children. Logistic regression analysis showed that the detection of IA-2Ab and IAA among Swedish children carries two and one and a half times disease risks respectively, as compared to non-Swedish children. This means that IA-2Ab and to a lesser extent IAA may be related to disease risk among Swedish children. This finding is consistent with HLA results, especially with IA-2Ab, which are known to be associated with DQ8.
The above findings collectively may suggest that non-Swedish children with different genetic heritage are at an increased risk of T1D when they are born in Sweden but their risk is still lower than that of the Swedes. Non-Swedish children who carry mainly low to moderate genetic risk may be exposed to certain factors in Swedish environment that increased their disease risk. The findings of this study, therefore highlights the importance of both genetic and environmental factors in determining the risk of T1D.
In conclusion
Country of origin is an important determinant of T1D and children who migrate from countries with low incidence into a ”diabetogenic” environment such as Sweden have higher disease-risk than their fellow citizens.
References
1. International Diabetes Federation (IDF) World Atlas of Diabetes, 3rd edition. [Online] 2006. [cited 2008 Mars 2]; Available from:
.. www.eatlas.idf.org
2. Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. The Lancet 2008;371:1777–82.
3. Schranz D, Lernmark Å. Immunology in diabetes: an update. Diabetes Metab Rev 1998. 14:3-29.
4. Serrano-Rìos M, Goday A, Larrad TM: Migrant Populations and the Incidence of Type 1 Diabetes Mellitus: an Overview of the Literature with a Focus on the Spanish-Heritage Countries in Latin America. Diabetes Metab Res Rev 1999;15:113–32.
5. Hjern A, Söderström U: Parental country of birth is a major determinant of childhood type 1 diabetes in Sweden. Pediatric Diabetes 2008;9:35–9.
6. Eliasson M, Bostro G: Major public health problems – diabetes. Scandinavian Journal of Public Health, 2006;34(Suppl.67):59–68.
|Upp|
